Cosmic Calendar
I'm a sucker for a good story, and modern science has a fascinating story to tell. Only recently have I begun to wholeheartedly listen to its story. And call me self-centered, but I love stories about me. I love to hear about my past and how I came into the world. Further, a childlike curiosity drives me to understand why the world is the way it is. Science has a barn burner of a story.
The effort to understand the universe is one of the very few things that lifts human life a little above the level of farce, and gives it some of the grace of tragedy.—Steven Weinberg
In recent centuries, we have teased out fragments of our origin story, a tale strange and vast. It is inextricably bound to the story of the origin of the universe, for the universe gave birth to us. If its story had been different, we would be different—if we existed at all. The story occurs on a timescale that is almost beyond human comprehension. We have become accustomed to think of history as a few thousand years after we learned to write, or perhaps a few million years beyond that. Perhaps the dinosaurs seem like deep history. This utterly pales in comparison to the real story. Human history is only the smallest part of the story. Even dinosaurs or callow newcomers on the universal stage. Words fail (as they often do) to convey understanding. I want to experience this story for myself, to get a small taste of the true proportions of history.
One way we have experienced our stories in the past is through rituals and festivals marked out on a calendar. Early calendars made sense of the yearly rhyme of season and flood. Within the yearly cycle, we placed holy days commemorating important events, important gods, rites of initiation, and the world's mythic creation. The yearly repetition increased our connection to our world and imparted a sense of continuity to our lives.
Someone's genius guided them to combine the great story of science with the calendar. The premise of the remix is simple: take the history of the universe from its beginning to the present day and condense it to the span of a single year. Mark milestones in the history of the universe on the calendar as they happen at that reduced scale.
I first saw Carl Sagan present the Cosmic Calendar as part of his wonderful Cosmos series.1 I loved flying with him as a child in his ship of the imagination. He introduced me to the beautiful and fascinating world around me as seen through the curious, playful, shrewd eyes of scientific inquiry. His Cosmic Calendar is an excellent example of how thought provoking he was as a educator. He is missed.
He presents the Cosmic Calendar masterfully and humanely, and it still inspires me. Scientific understanding has progressed since he recorded that program. For example, scientific consensus tells us that the universe is most likely to be about 13.7 billion years old rather than 15 billion, and the Milky Way is thought to have formed much earlier than Sagan stated.
I have decided to update and extend the original Cosmic Calendar and to to follow the Cosmic Calendar for a year. Rather than just reading about our history, I wanted to experience it in a modern ritual. It's one thing to read about something or see it illustrated in a diagram; it's another thing entirely to experience the long year and watch as milestones pass by. When something happens on the Cosmic Calendar, I'll post about it and give some background, maybe suggesting some places to investigate further or ways to observe the holiday.
At the time scale of a revised Cosmic Calendar:
1 year = 13.7 billion years
1 month ≈ 1.1 billion years
1 day = 37.5 million years
1 hour = 1.5 million years
1 minute = 26,000 years
1 second = 434 years
0.16 seconds ≈ 1 modern human lifetime
I can't get over the fact that my life is literally less than a blink of the eye on the Cosmic Calendar. How ephemeral am I! While I am saddened by the relatively short duration of my life, I am awestruck by the vastness of time.
If you would like to follow along, it may help to subscribe to my version of the Cosmic Calendar (XML or iCal).2
Caveat lector
I am not an expert on any of the materials included in the calendar, only an interested layman. It is highly likely that I will make mistakes in compiling the calendar. I will cite my sources—too many from Wikipedia I suspect—and endeavor to improve the calendar as time goes on.
Also note that science operates on consensus. The corollary to that is there will always be disagreement at the limits of science. I have tried to harmonize any conflicting information that I have found, but in the hands of a hobbyist, the nuances of the scientific debate is sure to get mangled.
I could have renamed this the Human Advent Calendar because this is the story of our coming into the world. It begins to answer the questions "Who am I?" and "Where did I come from?" from a human perspective. It may be self-centered, but as I said, I like stories about me. However, this shouldn't be taken as an endorsement of the idea that homo sapiens is the culmination of creation. It seems perfectly clear that we are just another wayfarer in the epic tale of this universe. The rest of the universe has just as much claim as we to the title of center of the universe.
As a last warning, science moves on. This calendar, even where it fairly represents current scientific understanding, should not be taken as dogma. If new data come in that conflict with the calendar, out with the old, in with the new; no regrets.
Further Study
Maps of Time: An Introduction to Big History by David Christian
The Structure of Big History: From the Big Bang until Today by Fred Spier
Big History: From the Big Bang to the Present by Cynthia Stokes Brown
Big History: The Big Bang, Life on Earth, and the Rise of Humanity (lectures) by David Christian
1. It was also published in his book The Dragons of Eden.
2. Anyone who wants to verify my dates can check the source code for the script I wrote just for this purpose. I sometimes used a calculator and a day-of-year table as a sanity check.
01 Jan—Big Bang
Big Bang — 1 January, 12:00 midnight
In its beginning, everything was a single point. This cosmic womb nurtured everything before it came to be. Every star; every planet; every flower; every lion, tiger, or bear; every Mozart, Einstein, or Madonna; every banana split, parfait, or brownie à la mode; every Illiad, I-Ching, or Bible; every hatred, joy, or love; every thing lay dormant within this primordial point. This is the story of how that point became our world, became us. This is our story.
According to current theory, all of what currently makes up the universe was packed into a space no larger than an atom. It doesn't make sense to ask what was outside that point because even space was curled up inside this cosmic egg. It's kind of like asking what's north of the North Pole. Nor does it make sense to ask what happened before the point began to expand billions of years ago because time began its flow with the expansion. Our universe began in the in what is known as the Big Bang.
There was neither non-existence nor existence then. There was neither the realm of space nor the sky which is beyond. What stirred? Where? In whose protection? Was there water, bottlemlessly deep?
There was neither death nor immortality then. There was no distinguishing sign of night nor of day. That One breathed, windless, by its own impulse. Other than that there was nothing beyond.
Darkness was hidden by darkness in the beginning, with no distinguishing sign, all this was water. The life force that was covered with emptiness, that One arose through the power of heat.
Desire came upon that One in the beginning, that was the first seed of mind. Poets seeking in their heart with wisdom found the bond of existence and non-existence.
Their cord was extended across. Was there below? Was there above? There were seed-placers, there were powers. There was impulse beneath, there was giving forth above.
Who really knows? Who will here proclaim it? Whence was it produced? Whence is this creation? The gods came afterwards, with the creation of this universe. Who then knows whence it has arisen?
Whence this creation has arisen—perhaps it formed itself, or perhaps it did not – the One who looks down on it, in the highest heaven, only He knows or perhaps He does not know. (Nāsadīya Sukta, Rigveda)
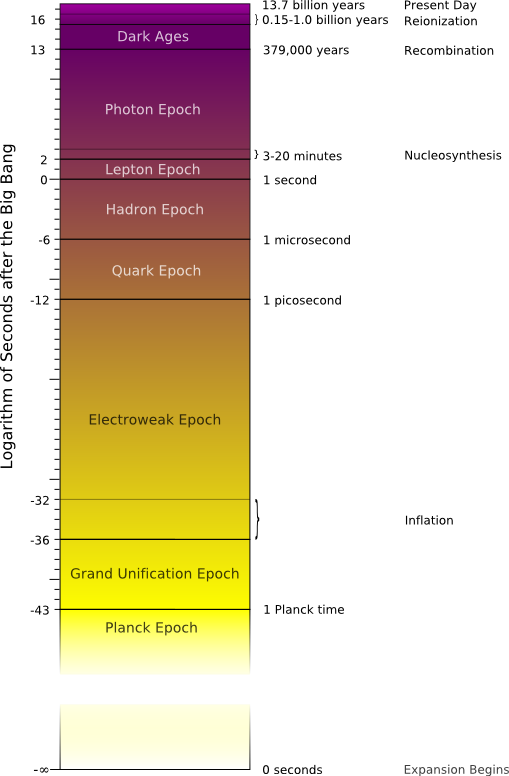 Planck Epoch: We really don't know what was happening in the first instant of the Big Bang 13,700 million years ago (Mya). The laws of physics as we know them break down during the Planck Epoch, the first 10-43 seconds after the Big Bang.1 This notation means one tenth multiplied by itself 43 times, or put another way, 0.000 000 000 000 000 000 000 000 000 000 000 000 000 000 1, an extremely, extremely small number. According to one theory, the universe was about 10-35 meters across.2 Because all of the matter and energy of the universe were packed into such a small space, it was also ludicrously hot: 1032 degrees Celsius. This notation means 10 multiplied by itself 32 times, or 100 000 000 000 000 000 000 000 000 000 000.3
Planck Epoch: We really don't know what was happening in the first instant of the Big Bang 13,700 million years ago (Mya). The laws of physics as we know them break down during the Planck Epoch, the first 10-43 seconds after the Big Bang.1 This notation means one tenth multiplied by itself 43 times, or put another way, 0.000 000 000 000 000 000 000 000 000 000 000 000 000 000 1, an extremely, extremely small number. According to one theory, the universe was about 10-35 meters across.2 Because all of the matter and energy of the universe were packed into such a small space, it was also ludicrously hot: 1032 degrees Celsius. This notation means 10 multiplied by itself 32 times, or 100 000 000 000 000 000 000 000 000 000 000.3
It is impossible to fully comprehend how extremely small the universe was. To try, imagine a young child about one meter tall who holds in their cupped hand a sphere that is 10-35 meters across. Actually, the sphere is so small that the child's hand would appear empty. Now, stretch the child and the sphere until the child is as tall as the diameter of the universe that we can currently see. Light travels very fast—186,000 miles per second—but it would take 93 billion years for light to travel from the child's head to its foot. The sphere has stretched too, but if we were cradled in the child's gigantic hand, the expanded sphere would still be too small for us to see. It would still only be a few atoms wide. This is unimaginably small.
Just as the universe was incomprehensibly small, the temperature was incomprehensibly large. For comparison, the core of our sun is only 107 degrees Celsius (i.e. 10,000,000 degrees). Multiply the heat of the sun by ten million. Hellish, we might be tempted to call it. Now multiply that hellish temperature by another million. And do it again. And again. And again. That is wicked hot!
All of the fundamental forces which govern our universe—gravity, electromagnetism, and the strong and weak nuclear forces—were equally strong and acted as one during the Planck Epoch. Today, gravity attracts all matter together and is the force that keeps the Earth circling the Sun and our feet firmly planted on the ground. The electromagnetic force governs light and magnetism. It makes radio, television, and cell phones possible and it holds our atoms together. The nuclear forces govern interactions within the nucleus of atoms. In the beginning, they were a single force.
Grand Unification Epoch: At the end of the Planck Epoch, an unimaginably small moment in time, this symmetry broke and gravity became weaker, separating itself from the other forces. (Please refer to the time line.)
As the universe expanded, it cooled down. But at this early stage immediately after the symmetry of universal forces was broken, the universe was still incredibly hot: 1027 degrees Celsius.
The Grand Unification Epoch is so named because the nuclear forces and the electromagnetic force were still unified in a single force called the electronuclear force. This epoch ended 10-36 seconds after the Big Bang when the strong force broke away from the others.
Electroweak Epoch: When the strong force separated from the others, the universe had cooled to about 1015 degrees Celsius and began a period of incredible expansion known as cosmic inflation. Its diameter increased in size by a factor of about 1026 in a small fraction of a second: by the end of this epoch something that had been the size of a millimeter grew to dwarf the Milky Way galaxy. Elementary particles were stretched to cosmic sizes, all within 10-32 seconds. Big Bang indeed.
Quark Epoch: This period began 10-12 seconds after the Big Bang when the electromagnetic and weak forces separated themselves and the four fundamental forces took their present form. The universe had cooled enough that subatomic quarks and gluons—the basic building blocks of matter—could condense out of its roiling energy. The universe was still too hot, however, for quarks to bind to each other to form neutrons and protons.
Hadron Epoch: One microsecond after the Big Bang, the universe had cooled enough to allow quarks to form hadrons such as protons and neutrons, the building blocks of the nuclei of all atoms. A nearly equal number of particles and anti-particles were forged from quarks in the primordial furnace. Particles and anti-particles have an explosive relationship. When they collide, both are annihilated in an explosion that releases tremendous energy. At this point, any hadrons that were destroyed in this way were replaced by others that were created in the heat of the early universe.
The universe continued to cool, reaching the point where hadrons were no longer being created. Most of the particles and anti-particles soon destroyed each other. When the figurative smoke cleared, all of the anti-hadrons were destroyed, but a small number of hadrons were left over. You and I and everything we see are partly made of those leftover hadrons. We exist because of an imbalance in particle/anti-particle destruction.
Lepton Epoch: One second after the Big Bang when most of the hadrons and anti-hadrons had destroyed each other, leptons (such as the familiar electrons) dominated the mass of the universe. The universe was still creating pairs of leptons and anti-leptons until three seconds after the Big Bang. In a now familiar story, most of the leptons and anti-leptons destroyed each other, but a small residue of leptons survived (to later create atoms later in the story).
Photon Epoch: After most of the pairs of leptons and anti-leptons had destroyed each other, photons—particles of light—made up most of the energy in the universe. Photons were still being scattered by electrons. The universe was therefore opaque: light couldn't shine through the thick soup of scattering particles. Protons and neutrons began to form small atomic nuclei (e.g. helium, lithium, and beryllium).
Matter Domination—1 January, 12:03 AM
70,000 years after the Big Bang (3 minutes at the scale of the Cosmic Calendar), the amount of what we would call matter had grown to become equal to the amount of radiation (e.g. light) in the universe.
Recombination and Dark Ages—1 January 12:16 AM
Up to about 379,000 years after the Big Bang, the universe was filled with a plasma. In other words, the electrons were racing around unattached to atomic nuclei (contrary to our normal experience). It was just too hot for electrons to settle down. Lightning and the sun are two common examples of plasmas. Light is scattered by plasma, so light couldn't travel very far in a straight line in the early universe. You could say that visibility was practically zero. If you were alive then (and could manage to stay alive), you wouldn't be able to see the end of your nose.
After 379,000 years, the universe had cooled enough to allow atomic nuclei to capture electrons and form true atoms such as hydrogen and helium. The fancy name for this is recombination. This freed the light that had been held captive by the universal plasma. The universe became transparent; visibility was no longer zero. The consequent burst of light as the universe became transparent is known as the cosmic microwave background.
After the release of photons, the universe was plunged into darkness. No new light was being generated. This was the beginning of the Dark Ages.
Observance Ideas
- Watch a fireworks show at the stroke of midnight and think about cosmology.
- Make your own big bang (wink wink) at midnight… while thinking about cosmology.
Further Study
A Brief History of Time by Stephen W. Hawking
The Universe in a Nutshell by Stephen W. Hawking
Born With a Bang by Jennifer Morgan
Character of Physical Law by Richard Feynman
QED by RichardFeynman
1. This unit period of time is known as a Planck time after Max Planck, the founder of quantum theory.
2. A unit of length known as the Planck length.
3. A unit of temperature known as the Planck temperature. Note that I use the Celsius scale because it is more familiar than the Kelvin scale to most readers. Even though the Kelvin scale is technically more correct, at these temperatures the difference is negligible anyway.
05 Jan—Galaxies and Stars

And the earth was without form, and void; and darkness was upon the face of the deep. (Genesis 1:2)
One billion years have passed on the Cosmic Calendar since the Big Bang. Much of that time has been spent in the darkness known as the Dark Ages. The universe created no new light since the time that the photons that were released during Recombination, the time when the primordial plasma of electrons and atomic nuclei coalesced into a sea of hydrogen and helium atoms. The universe continued to expand and cool. From this beginning, we might expect the universe to end in frozen darkness, yet when we look to the heavens we see myriad stars, blazing stellar furnaces.
What happened to change the cold, dark fate of the universe?
The early universe was also very homogeneous. The matter and energy in the universe was spread out smoothly, almost perfectly so. Small fluctuations did exist,1 yet the universe was so smooth—How smooth was it?—it would be like hiking the 4,500 kilometers (2,800 miles) from Los Angeles to New York in an alternate universe where the terrain was flatter than Kansas, like glass. The only landmark to relieve the monotony of the months of hiking across glassy plains would be a single blip of a hill perhaps seven stories tall.
Yet the universe isn't so plain and boring now. Everywhere we look we see a riot of diversity and complexity. How did unrelenting plainness become practically infinite diversity?
The Dark Ages weren't an uneventful chapter in our story. Things were taking shape in the darkness.
Reionization—5 January 2009
Some of the answers to our questions may lie in the colossal inflation of the universe that happened during the Electroweak Epoch. If inflationary theory is correct, those tiny fluctuations in the early universe became the seeds for the structure that we see today. When the universe began to inflate to about 1026 times its size, these quantum fluctuations smaller than atoms got caught up in inflation. They grew to galactic proportions and started in motion the formation of structure.
In its early history, the universe was dominated by the expansion of the Big Bang. As time went on, another force began to assert itself at large scales: gravitation (gravity), the force that attracts all particles of matter to each other. Inflated quantum fluctuations created some places of greater density where more matter was packed into a small space. Because these places of greater density had more matter, they had greater gravity. Because they had greater gravity, they could attract even more matter. An so on.
In the inky darkness, the first structures began to take shape.
The first structures to form (about 13,550 million years ago) were what we see today as galaxy clusters. Dense clouds of hydrogen and helium gas and dark matter collapsed in on themselves due to the force of their own gravity. Like a runaway train, nothing was yet able to stop this collapse.
Galaxies and Quasars
Smaller parts of these huge clouds were denser than others and began the process of gravitational collapse at a smaller scale. As the clouds of atoms and dark matter collapsed, they began to spin, forming the first nascent galaxies, including our own Milky Way.2 As atoms fell into these swirling vortices, they collided with each other. These collisions created heat. The universe began to warm up again.
At the heart of most large galaxies is a supermassive black hole 105 to 1010 times as massive as the sun.3 A black hole is a region of space that is so dense that its tremendous gravity captures everything that comes too close. Even light is unable to escape its gravity once it gets too close.
These monsters had a voracious appetite, consuming tremendous amounts of matter in the early universe. As the black hole eats, it is thought to create very dense regions where the infalling matter has been packed together very tightly. This somehow releases tremendous energy and light. These bright phenomena are known as quasars, the brightest objects in the visible universe. The brightest quasar in our sky would be as bright as the sun if it were 33 light years away, almost 2 million times as far away as the sun. This particular quasar is therefore about 2 trillion times as bright as the sun or 100 times as bright as the average galaxy!
Of the 100,000 known quasars, the nearest to us is 780 million light years away. Most of them are much farther. Quasars were therefore more common in the early universe. Once they run out of matter to consume, quasars turn off. It seems that the age of quasars is over.
Stars
Parts of these galaxies were denser than others, so the process of collapse repeated itself yet again at smaller scales. Clouds of gas inside galaxies began to collapse in on themselves and spin just like their parent galaxies had. These spinning clouds of gas were the beginnings of the first stars. As they collapsed, they generated even more heat, enough to strip the electrons from the nuclei of atoms. These proto-stars eventually got dense and hot enough that the bare atomic nuclei began to fuse together to form heavier elements when they collided. When nuclei fuse together, some of their mass is lost and is converted directly into energy and light. Nuclear fusion is what lights the stars.
The stellar furnaces had been lit.
Nuclear fusion is also what finally stops the progress of gravitational collapse. Stellar radiation and heat exerts an outward pressure that balances against the inward pull of gravity. This dynamic equilibrium holds stars together and prevents them from collapsing altogether.
The radiation from newly formed stars began to heat up the gas between the stars. These gaseous atoms also lost their electrons and became ions again. The atoms were ions when they first formed, before they trapped electrons during Recombination. Today, almost all of the visible matter in the universe is ionized. For this reason, this period of reheating is known as Reionization, the time when the universe lit itself again and came out of the Dark Ages.
Stelliferous Age—27 January
On the Cosmic Calendar, the first creation of galaxies and stars lasted until about 12,700 million years ago. That is how the cosmos came to have the shape and light that we see.
The next stop in our story happens at the end of August.
Observance Ideas
- Go stargazing. Although it is preferable to do this beyond the pollution of city lights, it isn't necessary to have an awe inspiring moment contemplating the vastness of our universe.
1. These were on the order of 1 part in 100,000.
2. The age of the Milky Way is based on the age of Cayrel's Star.
3. That is 100,000 to 100,000,000,000 times as massive as the sun. The sun is 332,950 times as massive as the Earth.
31 Aug—Mother Star

"I believe a leaf of grass is no less than the journeywork of the stars,…"
— Leaves of Grass
Two thirds of the age of the universe has passed since the last date marked on the Cosmic Calendar, but the universe has not been idle. It has been hard at work creating the elements that made our evolution possible.
The Big Bang ended too quickly to create the complex atoms necessary to build our world. It created only hydrogen and helium, the two lightest elements, with only traces of heavier elements. Human life relies on the complex combinations of elements which the more complex make possible. With a simple palette of two elements, we could never have evolved.
The stars which formed after the Big Bang fueled their furnaces by fusing together the simple elements. Two hydrogen atoms, for example, could fuse together to create a single helium atom. In the process, the atoms released the energy that heated and illuminated the stars.
The energy released in these atomic fusion reactions also prevented the stars from collapsing. It exerted an outward pressure that balanced the inward pressure of gravity.
These earliest stars were giants and lived short, violent lives. As they aged, they exhausted their cores' hydrogen fuel. As a star exhausted its fuel, its furnace cooled and the outward pressure that prevented its gravitational collapse began to fail. The star's core would begin to collapse.
As the core collapsed, the star's atoms packed closer and closer together causing the temperature and pressure to increase. At sufficiently high temperatures and pressures, helium can fuse together. As the pressure and temperature reached this threshold, the core would reignite with with helium fusion reactions instead of hydrogen.
The star would begin forming carbon (and a few other elements such as calcium, sulfer, and magnesium) at its core. This new fusion reaction released enough energy and outward pressure to halt further collapse.
Even though hydrogen had been exhausted in the core, hydrogen fusion continued in an outer layer of the star. The star became layered with a helium fusing core and a hydrogen fusing outer layer.
Eventually, the star would run low on helium in its core, and the process of collapse, increasing pressure, and reignition would be repeated. This time, carbon atoms would begin to fuse into neon. This process repeated several times until the star is layered like an onion with an iron-nickel core surrounded by layers of silicon, oxygen, neon, carbon, helium, and hydrogen in that order. ((The nickel is formed through fusion and later decays into iron.))
When the star exhausted its silicon fuel, it would run out of options. The fusion of nickel releases no energy. Instead it absorbs energy, so this reaction could halt another core collapse. When the core runs low on silicon, the core begins to collapse again, but the star is powerless to forestall its imminent death.
The collapse of the core would create conditions favorable for the creation of elements heavier than iron. It would collapse in on itself as fast as 70,000 kilometers per second, about one-fourth the speed of light. This collapse would form a solid core of neutrons. (Stars greater than twenty times the mass of the Sun would go on to collapse into a black hole.)
After the formation of the neutron core, the star would explode in what is known as a supernova. During this explosion, it is possible to create atoms heavier than iron and nickel. A significant portion of these extra heavy elements like gold, uranium, and lead were formed during supernovae.
The supernova explosion would throw off a shock wave of material into the interstellar void, broadcasting its heavy atoms and leaving behind the dense, collapsed core. The shock wave would perturb the inert material between the stars and trigger the formation of new stars in its wake.
A new generation of stars grew from the materials that the supernovae of the first stars left in their wakes. The new generation started their lives with heavy atoms gleaned from the previous generation. The new generation continued the work of fusing together light atoms to make heavier atoms.
Not all stars end in supernovae, but when those of the new generation massive enough to create a supernova died, they too broadcast their heavy elements into the universe and the cycle of stellar life began anew. Each successive generation of stars has had an increasing concentration of heavy atoms formed by the previous generation.
Thus we owe the heavier atoms essential for our existence — such as oxygen, carbon, nitrogen, calcium, and phosphorus — to the alchemy at the heart of stars that have long since died.
Mother Star Supernova—31 August
We believe that our Sun got its start when a star somewhere in the Milky Way galaxy went supernova. About 4,600 million years ago, this unknown supernova — our Sun's mother star — came to the end of its life.1, 2 The shock wave of its death gave birth to our own star, the Sun. Our bodies and almost everything we see are made of the materials that the mother star broadcast into the galaxy.
Further Study
Maps of Time by David Christian
Born With a Bang by Jennifer Morgan
1. Which supernova started our solar system? We don't know. The sun has traveled around the center of the Milky Way galaxy 20–30 times since it formed. Things have become so scrambled since then that we have no way of knowing where the sun started in the galaxy.
2. There may have been more than a single supernova that played a role in creating our solar system.
01 Sep—Sun

Just like all of the stars that came before it, our Sun began its life as a cloud of interstellar gas and dust.
The gravity of the atoms in the gas cloud — mainly hydrogen and helium — tried to pull the cloud together, but the faint pressure of the cloud prevented its collapse.
Then shock waves from our mother star's supernova disturbed this balance. They spread through the cloud creating eddies and pockets of denser gas in their wake. In the part of the cloud that would become our Sun, one of these slightly denser eddies began to collapse and spin. The shock waves had packed the atoms closely enough that gravity could overcome the pressure in the cloud.
In a now familiar dance, as the cloud contracted, it began spinning faster. In the center of this swirling cloud, the beginnings of of a star (a protostar) took shape and began to heat up as its atoms collided with each other.
Sun—1 September
When the protostar grew hot enough, it began to fuse its hydrogen into helium, becoming a fully fledged star about 4,570 million years ago. The energy released by these fusion reactions balanced the gravity of the star, its gravitational collapse halted, and our Sun became stable.
A disk of gas and dust that would soon become the planets of our solar system began forming around our newly born Sun.
02 Sep—Earth and Planets

The planets of our solar system began to form about 4,540 million years ago from dust and gas orbiting the newly formed Sun.
Solar System—2 September, 1 AM
As the dust particles collided with each other, they stuck together to form larger and larger clumps, like a snowball gathering snow.
Our young Sun produced a strong stellar wind (the solar wind), a steady stream of particles that it sent outward into interstellar space, the space between the stars.1 The solar wind heated the gas and dust and pushed it into interstellar space. After 3–10 million years, the solar wind had pushed all of the excess gas and dust out of the region where the planets were taking shape, and the planets stopped growing by accretion by about 100,000 years after the formation of the Sun.
Now, the planets could only grow by merging together the surviving planetesimals which had grown as big as one twentieth the size of the present-day Earth.
The solar wind made the inner solar system near the Sun too hot for ice. It scoured the inner solar system of its volatile chemicals that would easily vaporize, like water and methane. Because of the intensity of the young Sun's solar wind, only rocky, metallic planetesimals with no atmospheres or water remained in the inner solar system where we find the rocky planets today.
As the volatile chemicals traveled farther from the Sun, they got colder. Eventually, they reached a point where it was cold enough to become ice again, the frost line. As they collected outside this line, a planetesimal scooped them up. The abundance of volatile materials at the frost line allowed that planetesimal to become the largest planet in our solar system, Jupiter.
Because of its large mass, Jupiter was capable of capturing hydrogen and helium, the lightest elements and the most easily blown away the solar wind. Hydrogen and helium currently make up at least 87% of the mass of Jupiter. Because of its large atmosphere, it is known as a gas giant.
Saturn, another gas giant, formed farther from the Sun, and therefore had to take second helpings of the volatile chemicals caught up in the solar wind. It consequently never reached the same size as Jupiter, but it still became large enough to capture hydrogen and helium and become a gas giant.
Farther out still, two icy gas giants formed: Neptune and Uranus.
In the inner solar system, 50-100 planetesimals orbited the Sun. Each was between the size of the Moon and of Mars. Because the solar wind had swept the smaller particles of dust out of the solar system, the only way for these planetesimals to grow was by colliding and merging together. Over the next 100 million years, these violent collisions lead to the four rocky planets we know today: Mercury, Venus, Earth, and Mars.
Earth—2 September, 1 AM
The young Earth grew as planetesimals collided with it and they merged together. Gravitational compression, radioactive decay, and meteorite bombardment heated the Earth enough to melt its iron and nickel. These dense liquid metals sunk toward the core and lighter rocky materials floated to the surface.
Because of the intense pressure at the center of the Earth, a solid iron and nickel core formed. This core was surrounded by iron and nickel that were still liquid, then a mantle of liquid iron and magnesium silicates, and finally the lighter potassium and sodium silicates that later formed the crust.
This flowing core of liquid metal created Earth's magnetic field. The Earth's magnetic field makes compasses work, creates the aurorae, and protects us from the solar wind. Without the magnetic field, the solar wind would have dispersed the Earth's atmosphere long ago.
Earth's first atmosphere contained hydrogen and helium from the cloud that formed the Sun and planets. The atmosphere also contained gaseous silica, the mineral that we find in sand. The Earth lost most of this first atmosphere because it was not large enough like the gas giants to retain the light elements hydrogen and helium through the force of its gravity.
The Earth's birth signals the beginning of the Hadean Eon, an informal division of the Geological Time Scale.
The Earth was a forbidding place during this early eon. The surface was still a molten sea of lava. The continents had yet to form.
We would have suffocated because the atmosphere contained none of the oxygen essential to our kind of life. As the Earth grew large enough to retain a stable atmosphere, it captured the gases escaping from the molten sea of lava. This first stable atmosphere mas mostly water vapor with some carbon dioxide and hydrogen sulfide2 and small amounts of nitrogen, carbon monoxide, hydrogen, methane, and inert gases. This thick blanket of gas kept the Earth warm at a time when he Sun was not as bright as it is now.
Moon—2 September, 9 AM
The Moon formed about 13 million years after the solar system, 4,527 million years ago.
The leading hypothesis for how Earth's natural satellite formed involves a collision with a Mars-sized planet named Theia, which may have formed near the Earth. When it grew too large, Theia set out on a collision course with Earth.
Because Theia was so close to the Earth and couldn't pick up much momentum, the collision was a moderate one. Theia's iron core merged with Earth's, but some of each planet's rocky mantle was ejected into orbit around the Earth. This ejected material quickly formed into the Moon which began its life as an ocean of molten magma at least 500 km deep. The Moon slowly cooled, crystallized, and became solid.
----
1. The Sun still has a stellar wind, but it's not as strong as it was back then.
2. Hydrogen sulfide is the gas that gives rotten eggs their distinctive odor and contributes to the unpleasant odor of flatulence
11 Sep—Oceans

The Earth currently has approximately 344 million cubic miles of water, but early in the Earth's history, the solar wind had scoured most of the early Earth's water away, leaving the Earth rocky and barren. Some water trapped beneath the Earth's surface would have escaped into the atmosphere through volcanoes and outgassing, but not enough to explain the amount of water we have in our oceans today. Where did all the water that fills our oceans come from?
The early solar system was a wild place, full of roaming comets and asteroids that often crashed into their larger cousins, the planets. The leading theory about the origin of the oceans speculates that icy comets bombarded the Earth in its early history, bequeathing to us their water (and also their carbon dioxide, ammonia, nitrogen, and methane). The water we drink today may have come from as many as one million comets impacting the Earth billions of years ago.
At first, the Earth was too hot for the water be in a liquid state. Instead, it evaporated into the atmosphere, where it made up perhaps 80% of the early atmosphere. The rest of the atmosphere was still carbon dioxide and hydrogen sulfide, with small amounts of nitrogen and other gases.
The Earth may have had liquid water as early as 4,400 million years ago. The surface was much hotter then, but the heavy water vapor and carbon dioxide atmosphere created much more pressure than we experience today. The atmospheric pressure would have allowed liquid water to exist at high temperatures, perhaps twice the current boiling point of water.
Oceans—11 September
Anzu rent the sky with his talons,…
the flood came forth.…
The deluge belowed like a bull,
The wind resounded like a screaming eagle.
The darkness was dense, the sun was gone,…
the clamor of the deluge.
—The Epic of Atrahasis
As the earth cooled about 4,200 million years ago, all that water that had been locked in the atmosphere as water vapor began to condense into liquid water and rain down on the surface of the Earth. There was so much water in the atmosphere that these torrential rains lasted for millions of years and filled up the oceans. (Maps of Time, p. 63)
As the oceans formed, the carbon dioxide in the atmosphere began to dissolve in the newly formed oceans. The greenhouse effect of the carbon dioxide which trapped heat lessened and the Earth began to cool off even further. The exodus of the water and carbon dioxide left behind an atmosphere of mostly nitrogen, more similar to our atmosphere today except that it lacked oxygen.
21 Sep—Life

How life began remains a mystery. The Earth began as a lifeless rock but ended teeming with life. How did life rise from non-life?1
Lepidus: Your Serpent of Egypt, is bred now of your mud
by the operation of your Sun: so is your Crocodile.
Anthony: They are so.
— Anthony and Cleopatra, Act 2, Scene 7
Ironically, until the rise of modern science, we wouldn't have asked this question. The everyday creation of life from non-life was commonly accepted in Christendom. People believed, for example, that mice came from soiled rags, maggots from putrid meat, and snakes and crocodiles from river mud.
So entrenched was this belief that when experiments began to show that all life comes from other life, other scientists and laymen alike defended the spontaneous generation of life as an obvious fact. It wasn't until Louis Pasteur's famous experiment in 1859 that the idea of ongoing spontaneous generation was laid to rest.
Omnia omnino animalia—etiam vivipara, atque hominem adeo ipsum—ex ovo progigni. (Absolutely every animal—including those who give birth to live young, including man himself—are born from an egg.) — William Harvey
But that gave rise to the question: if all life comes from other life, where did the first living thing come from? Life must have come from non-life at least once.
Many hypotheses have been put forward to answer this question (too many to cover in detail), but none have gained a consensus. The mystery remains. We are unsure whether life began in shallow pools of water, radioactive beaches, undersea volcanic vents, below the Earth's surface, or even in outer space. Very basic organic chemicals could have been created in any of these locations, and thereafter life could have arisen from these building blocks.
Most hypotheses rely on an atmosphere devoid of free oxygen. Free oxygen reacts strongly with organic molecules and would have destroyed these materials on the early Earth. Early life relied on an environment that would be deadly to us today.
One prominent hypothesis proposes that life began when RNA formed from those building blocks. Most of us are familiar with DNA, the molecule that stores our genetic information, but RNA may be less familiar. RNA is a very closely related molecule to DNA.
Unlike DNA with its famous double helix, RNA is usually single stranded. RNA can also replicate itself whereas DNA needs assistance from other molecules. Because DNA can't copy itself, it is unlikely that life began with DNA. It is thought that RNA could have preceded it as the beginning of life.
Unfortunately for RNA's career as our future genetic code, it is less stable than DNA. It couldn't link together in long chains that contained enough information to code for complex life.
Once molecules of RNA formed and began to multiply through replicating themselves, the fundamental requirements for life were met. At some point, RNA could have handed over the job of storing genetic information to DNA, but RNA stuck around to help DNA reproduce.
But that is only one hypothesis among many, and it doesn't begin to address the question of how the genetic material got into cells.
However it happened, life had probably begun by about 3,800 million years ago—September 21st on the Cosmic Calendar—and made its way into cells by 3,500 million years ago. The earliest fossilized life is found in clumps of bacteria called stromatolites.
Life had begun.
(Note that human evolution took much longer than 2 million years, contrary to what Alan Watts suggests in his lecture.)
1. The question of how life began is separate from the question of evolution of species. Organic evolution requires that life already exists and says nothing about how life began.
18 Oct—Photosynthesis

Many forms of life get their energy directly from the Sun in a chemical process named photosynthesis. They create their own food from sunlight. Cyanobacteria first learned this trick about 2,800 million years ago, the 18th of October on the Cosmic Calendar.
Actually, that's not entirely true. Photosynthesis probably began much earlier—about 3,500 million years ago, the 29th of September on the Cosmic Calendar—with bacteria that evolved to use hydrogen and sulfur or other chemicals to convert light into food. But unlike the photosynthesis that cyanobacteria developed much later, that early anaerobic form of photosynthesis didn't create oxygen.
The oxygenic form of photosynthesis that we can thank cyanobacteria for is particularly important to us because without it, we couldn't exist. Oxygen helps us to create and store enough energy to sustain our complex and fast-paced life. Without oxygen, life would be stuck in the slow lane.1
Life has only discovered oxygenic photosynthesis once. The fundamentals of the process have remained unchanged ever since: the photosynthesizer takes in molecules of carbon dioxide and water and absorb the energy of photons from sunlight. The end product is sugar (glucose) and oxygen. For the photosynthesizer, oxygen is a toxic waste product which is expelled from the organism.
Green algae and plants also photosynthesize today, but they didn't learn how independently. They came by this ability in a surprising way.
Rather than evolve photosynthesis for themselves, these organisms' ancient ancestors imprisoned cyanobacteria inside their own cells.2 The cyanobacteria continued to do the work of photosynthesis, producing food for their kidnappers.
Ever since that time long ago, some cyanobacteria have lived inside of green algae and plants. They have adapted to life within their hosts and formed a symbiosis with them, but they are still recognizable as separate organisms. They have their own genome that closely resembles that of their free-range cyanobacteria cousins and which gets passed down from generation to generation inside of algae and plant cells. They are known as the little organelles called chloroplasts inside of every green algae and plant cell.
Without this symbiosis, plants couldn't get their energy from the Sun and we wouldn't exist.
1. More will be said about this when we discuss the Oxygen Catastrophe.
2. We will discuss discuss this again when we come to the development of eukaryotes.
29 Oct—Oxygen Catastrophe

A catastrophe had been in the works ever since the discovery of oxygenic photosynthesis. The organisms who converted sunlight, water, and carbon dioxide into food and oxygen were slowly poisoning their planet. About 2,400 million years ago—October 29th on the Cosmic Calendar—the buildup of oxygen in the environment led to a crisis for life on Earth.
Oxygen was a deadly poison to early life. It reacts strongly with organic chemicals, burning them in a kind of slow fire.
At first, the iron in rocks absorbed the oxygen created by photosynthesis. The rocks sequestered the oxygen and kept life safe from the toxic waste, but after millions of years, the rocks couldn't store any more oxygen. Organisms were also being buried and taking their carbon with them where it couldn't react with free oxygen to become carbon dioxide. Over time, oxygen began to find its way into the environment, and the Oxygen Catastrophe began.
Life couldn't stay the same. It had to adapt to the environment it had created. It had to find a way to live on or face extinction. Many lifeforms died out. They had evolved in an environment without free-roaming oxygen and were unprepared to face this change. The Oxygen Catastrophe was the Earth's first mass extinction.
Other forms of life found a way to cope with their toxic planet. They adapted showing life's amazing talent for innovation. It not only survived, life turned this new environment to its favor and learned to thrive.
Oxygen remade the surface of the Earth and made complex life possible. It's hard to overstate the difference that oxygen has made in the history of life on Earth.
The biggest difference it made was in making more energy available. Large organisms could eat small organisms and get more out of their meal. Earlier forms of biochemistry were terribly inefficient. At each level of the food chain, 90% of the energy stored in an organism's body would be lost when eaten by another. Oxygenic biochemistry lost only 40% at each level. This allowed much more complex food chains, and much larger, more complex lifeforms. Life may never have become multicellular if cyanobacteria hadn't discovered oxygenic photosynthesis.
Oxygen also created a protective layer in the upper atmosphere, the Ozone Layer, which blocked harmful ultraviolet radiation from reaching the Earth's surface. This paved the way for the later colonization of the land which had been to dangerous for life up to that point.
Oxygen even prevented the oceans from evaporating. Without oxygen, ultraviolet radiation would occasionally split ocean water into oxygen and hydrogen. The oxygen might react with something else leaving the hydrogen (even lighter than helium) to float up through the atmosphere and escape into space. The oceans would have evaporated into space over the course of eons.
With free oxygen in the atmosphere courtesy of photosynthesizers, any hydrogen that escapes the ocean today will bump into an oxygen molecule and become water again. Instead of escaping the Earth, it falls back to the surface as rain.
Mars shows us what may have happened to Earth. Mars probably had oceans long ago. Without life that knew how to photosynthesize, Mars probably lost its oceans and became a rusty desert as the oceans escaped into space.1
As if all that weren't enough, oxygen is essential in the formation of lignin and collagen which give structure to plants and animals respectively. Without lignin to surround their cell walls, plants would be too weak to stand on their own. Collagen surrounds animal cells and is the primary component of cartilage, ligaments, tendons, bone, and skin. Without collagen, animals would have all looked like amoebas lacking any structure.
To top it all off, oxygen makes the sky blue.
Throughout the Oxygen Catastrophe, life showed resilience and innovation. What looked like an utter disaster turned out to also be an opportunity for life to grow in new and exciting ways.
Recommended Reading
1. Mars may have lost its oceans even if it had oxygenic photosynthesizers, because of its small size and consequently lower gravity.